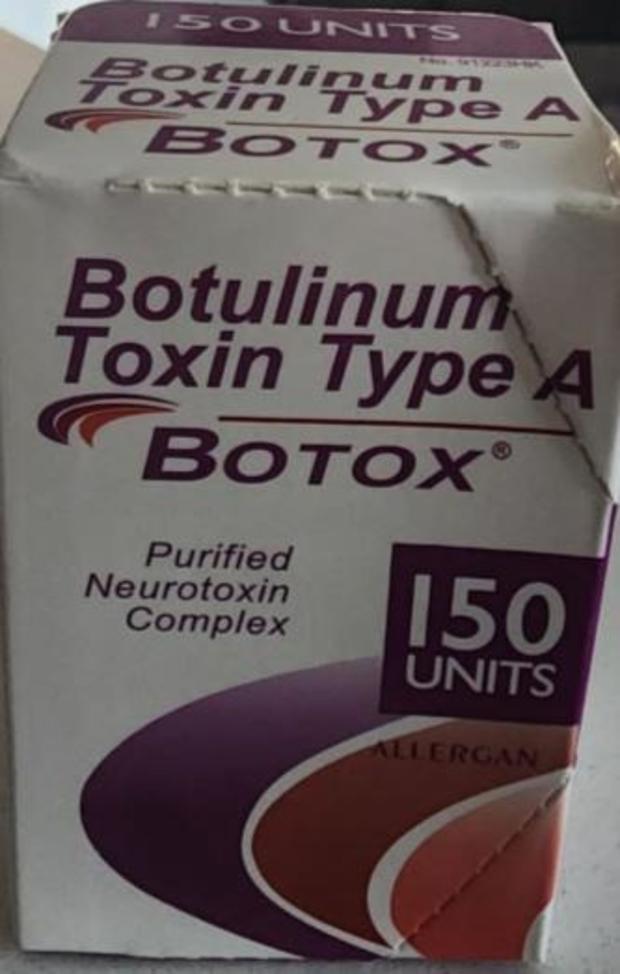
Dangerous counterfeit versions of botulinum toxin — better known as Botox — are being linked to an outbreak that has sickened 19 people in nine states, causing nine hospitalizations, federal safety officials are warning.
In a Tuesday alert to consumers and health care providers, the U.S. Food and Drug Administration said unsafe counterfeit versions of Botox had been found in multiple states and administered to people for cosmetic purposes.
The products “appear to have been purchased from unlicensed sources” and could be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe, the FDA said.
Two states — Illinois and Tennessee — last week reported half a dozen cases involving botulism-like symptoms following shots of potentially phony products. Since then, another 13 cases have been reported in an additional nine states, with all involving women injected with phony Botox by licensed and unlicensed individuals in non-medical settings, such as at homes or spas, according to the Centers for Disease Control and Prevention.
People reported experiencing botulism symptoms including blurred or double vision, drooping eyelids, difficulty swallowing, dry mouth, slurred speech, difficulty breathing and fatigue. The cases occurred in Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee and Washington.
U.S. Food and Drug Administration
The FDA is working with Botox manufacturer AbbVie to identify, investigate and remove suspected counterfeit Botox products found in the U.S. Currently, there’s nothing to indicate the illnesses are linked to the company’s FDA-approved Botox, with the genuine product safe and effective for its approved uses, the FDA noted.
U.S. Food and Drug Administration
In partnership with public health authorities, we have confirmed the security of our Botox and Botox cosmetic supply chain as well as the safety, quality, and efficacy of all products we manufacture and distribute,” AbbVie subsidiary Allergan told CBS MoneyWatch on Friday.
How to avoid counterfeit Botox
If you’re considering Botox for medical or cosmetic reasons, the CDC advises asking whether the provider, clinic or spa is licensed and trained to give the injections, and if the product is FDA approved and purchased from a reliable source. Some states have a look-up tool that can be used to check on licensing, according to the agency.
Those in doubt should not get the injection and those who experience symptoms of botulism should seek medical care or go to an emergency room immediately, the CDC said.
Approved for cosmetic use more than 20 years ago, Botox is a popular drug to smooth wrinkles and appear younger, with injections typically costing around $530, according to the American Society of Plastic Surgeons. The effects of a shot last three to four months on average, so additional shots are needed to remain wrinkle-free.
Federal officials have previously cracked down on unregulated Botox and other cosmetic treatments. In 2023, U.S. Customs and Border Protection officers in Ohio intercepted such fillers that had been shipped from Bulgaria, China, Korea and Spain.